Editor’s note: This text-based course is an edited transcript of the webinar, Respiratory Management of Patients With Neuromuscular Weakness Series: Review Recommendations Best Practice, presented by Dr. Duane Reed, EdD RRT, RCP. Any errors in transcription or editing are the responsibility of Continued.com and not the course presenter. It is recommended to download the course handout to supplement this text format.
Learning Outcomes
After this course, participants will be able to:
- Identify neuromuscular diseases that affect the respiratory system.
- Identify evidence-based recommendations for patients with neuromuscular diseases.
- Discuss best practice management for patients with neuromuscular diseases.
Overview of Neuromuscular Diseases (NMD)
Many forms of neuromuscular disease exist:
- Amyotrophic Lateral Sclerosis (ALS)
- Charcot Marie Tooth Disease (CMT)
- Multiple Sclerosis (MS)+
- Muscular Dystrophy
- Myasthenia Gravis
- Myopathy
- Myositis
- Guillain Barre Syndrome (not a disease, but when the immune system attacks the neuro system)
The foundation for our discussion comes from a panel discussion centered around 128 studies presented in the Journal of Chest. This panel has yielded 15 graded recommendations and a good practice and consensus-based statement. Our primary aim is to uncover evidence-based best practices for effectively managing the respiratory aspects of neuromuscular diseases.
Neuromuscular diseases manifest when the nerves governing movement are negatively impacted. This brings our attention to the nervous system. The resulting muscle weakness, a notable complication stemming from neuromuscular diseases, significantly affects the respiratory system. This connection becomes evident when considering the role of the diaphragm, a pivotal component of the respiratory system. The diaphragm relies on nerve impulses for stimulation. Thus, any damage to the nerves jeopardizes the function of the respiratory system. This is where the link between nerve damage and respiratory system vulnerability emerges. Respiratory failure is a prevalent complication closely linked to neuromuscular diseases.
This serves as a pivotal reason for us to delve into a discussion about various neuromuscular diseases. When nerve functionality is compromised, complexities can emerge, especially concerning respiratory function. This can lead to disruptions in the processes necessary for breathing and lung function. While you might be familiar with some of these conditions, others may be less known, yet all are intrinsically tied to the risk of respiratory failure. Understanding neuromuscular diseases' influence on the respiratory system is crucial because they often lead to respiratory complications due to their impact on nerves and muscles involved in breathing.
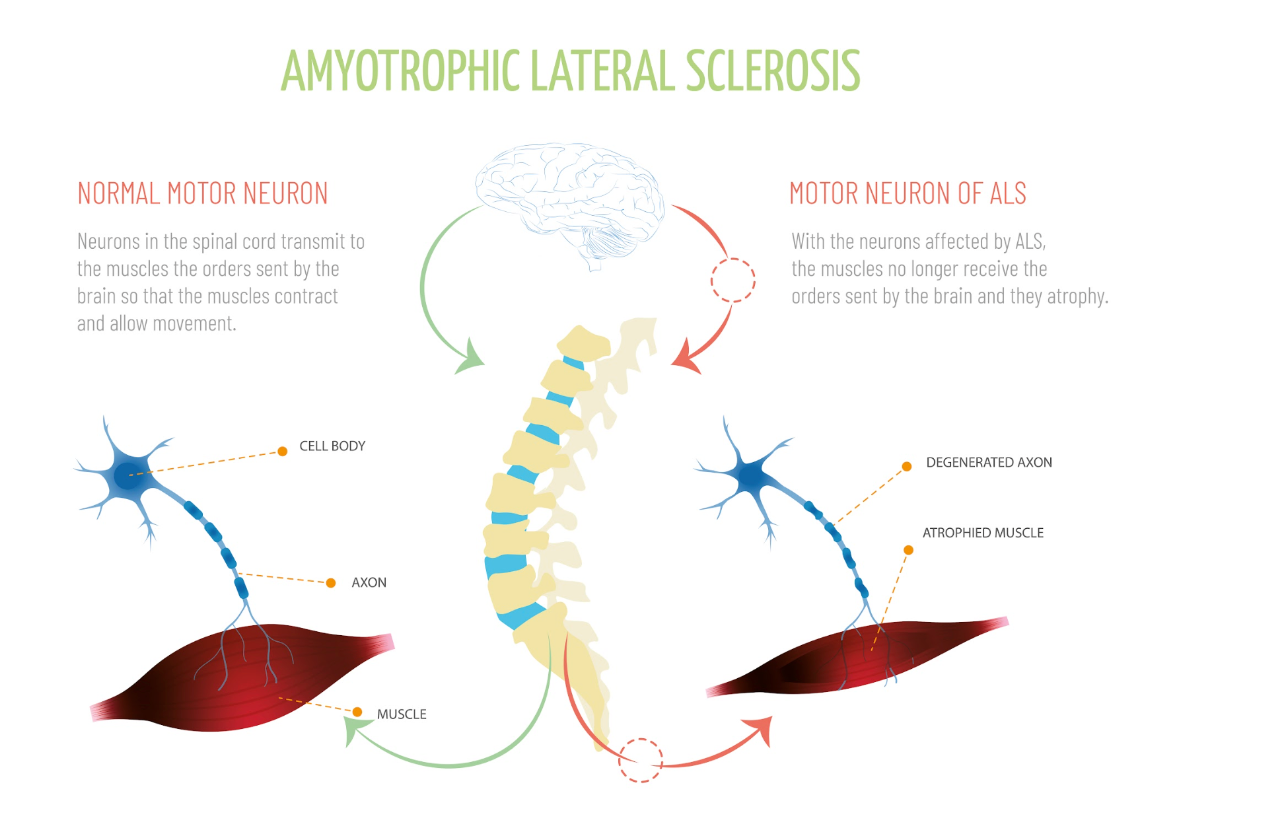
Figure 1. Diagram illustrating motor neuron of ALS (Continued Getty Images).
Many forms of neuromuscular diseases exist, with some being more familiar to us than others. One well-known example is ALS or amyotrophic lateral sclerosis in Figure 1. This disease primarily affects the nervous system, leading to a gradual weakening of the muscles. You may recognize it by its former name, Lou Gehrig's disease. It leads to muscle cell degeneration, resulting in a decline in muscle functionality and overall mobility.
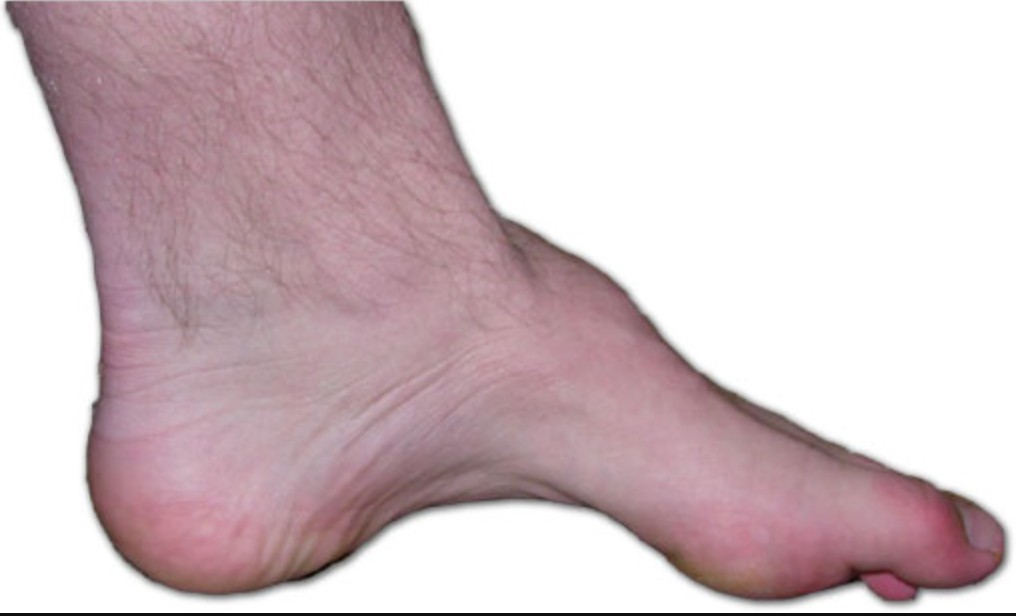
Figure 2. The foot of a person with Charcot-Marie-Tooth. (Benefros at English Wikipedia, CC BY-SA 3.0 via Wikimedia Commons).
Another example is Charcot-Marie-Tooth disease, commonly referred to as CMT. This condition is essentially a hereditary disorder that primarily induces nerve damage in the arms and legs seen in Figure 2. CMT disease leads to reduced muscle size and strength, accompanied by potential sensory loss, muscle contractions, and challenges in walking. Commonly observed are foot deformities like hammertoes and high arches. While symptoms often initiate in the lower extremities, progression to the hands and arms can occur over time. The manifestations of CMT disease generally emerge during adolescence or early adulthood, although they can also manifest in midlife.
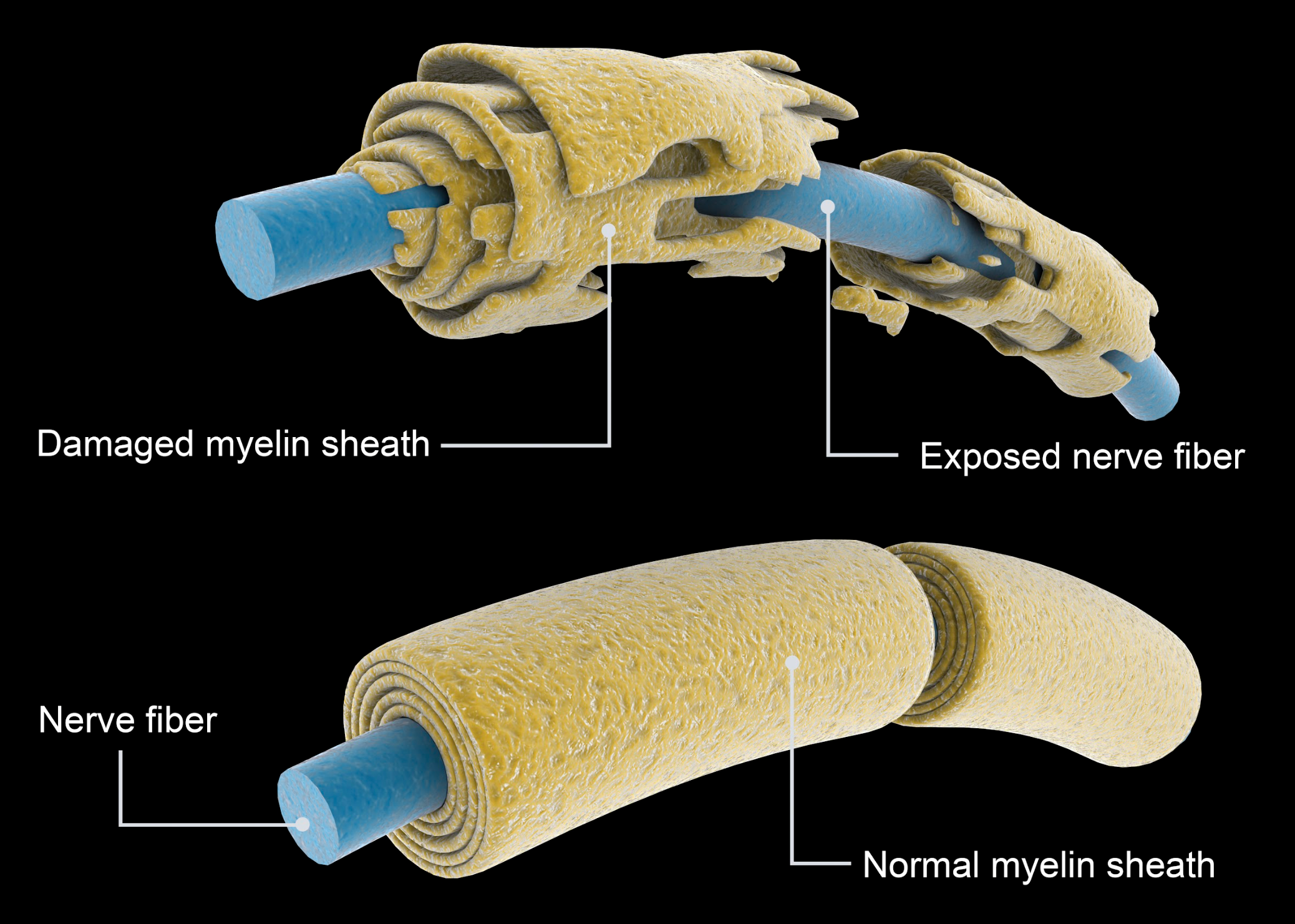
Figure 3. Damage myeline sheath of Multiple Sclerosis. (Continued Getty Images).
Multiple sclerosis in Figure 3, often referred to as MS, is a widely recognized condition. It is a potentially disabling disease affecting the brain, spinal cord, and central nervous system (CNS). In this disease, the immune system erroneously targets the protective sheath, known as myelin, covering nerve fibers. This leads to various issues, as the degradation of myelin disrupts the proper functioning of nerves, resulting in communication problems within the body. This condition can cause various symptoms and challenges, and we will provide an illustrative image to convey its effects better.
Muscular dystrophy is a widely recognized condition known for inducing muscle-related issues. This disorder also intricately affects the surrounding protective sheath of nerves. Over time, these issues inevitably extend their reach to the respiratory system, raising a pivotal concern. Delving into the context of ALS, a compelling image illustrates the contrast between a healthy motor neuron and one that is emblematic of ALS. These distinctions become apparent in the cell bodies and axons, both crucial for maintaining optimal nerve function. These transformations culminate in muscle atrophy, setting apart ALS-affected motor neurons from their healthy counterparts within the spinal cord. ALS is unquestionably a profoundly debilitating condition.
Moreover, we have previously explored Charcot-Marie-Tooth disease. A comparison between unaffected and affected nerves visually portrays the scarring of the myelin sheath, a distinctive feature of this hereditary disorder. The damage predominantly manifests in the arms and legs, impairing nerve and myelin sheath. This disease can significantly impair affected individuals, and its impact may even extend to lung function. It is important to note that while these diseases, such as multiple sclerosis, exhibit distinct origins, they share commonalities in terms of their repercussions.
Regarding multiple sclerosis, a parallel arises concerning the damage inflicted upon the myelin sheath. A visualization on the right effectively underscores the compromised state of the myelin, underscoring its pivotal role in safeguarding nerves. This assumes particular significance when considering nerves stemming from the cervical spine, which extend to the diaphragm. Any compromise in these nerves disrupts the necessary transmission for stimulating impulses, posing a considerable challenge to respiratory function. This emphasizes the significance of delving into neuromuscular diseases, given their substantial influence on the respiratory system, particularly within the realm of inherited disorders.
Among the array of disorders highlighted, myasthenia gravis emerges as a notable case. This condition typically initiates in the brain and progresses downward, impacting muscles and culminating in rapid fatigue. This importance cannot be understated, as it targets voluntary muscle groups, including those implicated in respiration. Conversely, while Guillain-Barre syndrome is classified as a syndrome rather than a disease, its effects on muscle function are noteworthy. Additionally, it is essential to distinguish myositis, characterized by inflammation of muscles, as a rare disorder. Both Guillain-Barre syndrome and myositis present substantial challenges.
All these diseases collectively manipulate nerve function. Once nerve integrity is compromised, the essential nerve impulses the diaphragm relies upon to generate pressure changes are disrupted. This underscores the potential complexities that can arise when nerve functionality is compromised, particularly concerning respiratory function.
Advancing Research on Respiratory Management of Patients with Neuromuscular Weakness
An expert panel conducted a systematic review focusing on the respiratory management of neuromuscular diseases (NMD). They employed the Grading of Recommendations, Assessment, Development, and Evaluations approach to assess the certainty of evidence, formulate recommendations, and assign grades. The consensus was reached through a modified Delphi technique.
Drawing insights from 128 studies, the panel established 15 graded recommendations, one good practice statement, and one consensus-based statement. It is important to note that the evidence supporting best practices for NMD respiratory management is limited, primarily relying on observational data from amyotrophic lateral sclerosis.
The guidelines offered an understanding of areas such as mouthpiece ventilation, the transition to home mechanical ventilation, salivary secretion management, and airway clearance therapies. It's emphasized that the pathologic features of NMD encompass a wide spectrum of disorders, each with varying rates of lung function decline. In clinical practice, the role of clinicians extends beyond medical evaluation to shared decision-making with patients and families. This involves respecting patient preferences and treatment goals, considering the quality of life, and judiciously utilizing available resources in decision-making processes.
Panel Recommendation 1: Use and Timing of Pulmonary Function Test (PFT)
In the context of patients with neuromuscular diseases at risk of respiratory complications, experts advise incorporating the timing of Pulmonary Function Testing (PFT) as a valuable tool for management. The panel strongly recommends the integration of PFT into the management process. These recommendations aim to serve as a basis for developing protocols and guidelines.
Panel Suggestions:
The panel proposes PFT as a cost-effective intervention. They recommend spirometry, focusing on FVC (forced vital capacity) and SVC (slow vital capacity), along with MIP (maximum inspiratory pressure), MEP (maximum expiratory pressure), and SNIP (nasal inspiratory pressure) measurements for patients with neuromuscular conditions. Additionally, peak cough flow should be considered. FVC and SVC techniques should be employed to assess respiratory health comprehensively. A slower version like SVC is crucial for patients with COPD who may experience prolonged air trapping. Evaluating respiratory reserve and muscle strength involves measuring MIP and SNIP. These parameters are indispensable when managing neuromuscular patients, expanding our toolkit. Panel recommendation two emphasizes utilizing PFTs and understanding their optimal timing.
Panel Recommendation 2: Use and Timing of PFT for Patients with NMD at Risk of Respiratory Failure
For patients with neuromuscular disease prone to respiratory complications. The panel advises conducting PFTs at least every six months, as deemed suitable. This recurring six-month interval aligns with the recommended frequency.
Panel Suggestions:
Employing a consensus-based approach, the panel arrived at a six-month testing interval. While recognizing that the progression rates vary among NMDs, particularly evident in cases like ALS, where significant respiratory parameter changes could manifest in 3 to 6 months, the panel acknowledged that for more stable or slowly progressing conditions like Duchenne muscular dystrophy, PFTs could be conducted at less frequent intervals, such as every 12 months. The panel proposes considering one or more of the following assessments within this timeframe. While not mandatory to perform all, options include measuring vital capacity, slow vital capacity, MIP, MEP, SNIP, and cough.
Panel Recommendation 3: Screening for Respiratory Failure and Sleep-Related Breathing Disorders
Let's delve into recommendation three, which revolves around sleep-related disorders in the context of neuromuscular diseases. When working with patients with symptomatic patients with NMD who have normal PFT, the panel proposes employing overnight oximetry, often referred to as ONO. Additionally, the suggestion involves utilizing polysomnography to evaluate the necessity for non-invasive ventilation. This step relates to the consideration of sleep studies as well.
Panel Suggestions:
The panel highlights polysomnography as a valuable tool for determining non-invasive ventilation's appropriateness. Clinicians, consider polysomnography to assess whether noninvasive ventilation (NIV) is clinically indicated. This particular ventilation approach includes methods like BiPap.
Panel Recommendation 4: Noninvasive Ventilation (NIV)
Moving on to recommendation four primarily centers around patients with neuromuscular diseases experiencing chronic respiratory failure. This scenario necessitates a closer look at non-invasive ventilation strategies. When discussing chronic respiratory failure, it is important to note that this often pertains to COPD patients.
Panel Suggestions:
The panel's recommendation of employing non-invasive ventilation aligns logically with this context. However, it is worth highlighting that while clinical indications for non-invasive ventilation are present, they can vary based on factors such as the specific neuromuscular disease, patient age, and the pace of disease progression. In essence, these multifaceted variables influence the suitability of non-invasive ventilation.
The evidence was primarily derived from adults with ALS, given its prevalence as the most common NMD. However, its application should be approached thoughtfully when considering younger children, adolescents, and adults with non-ALS diagnoses. The panel further suggests that its applicability remains subject to these diverse factors even with established indications for non-invasive ventilation. The panel highlights key indicators, such as a decrease in forced vital capacity (FVC) to less than 80% of predicted accompanied by symptoms or an FVC below 50% of predicted symptoms. Similarly, a SNIP or MIP measurement indicative of a reserve of -40 centimeters of water can signify the need for non-invasive ventilation.
The guidelines have transitioned from recommendations and marked a significant development in 2023. Specifically, if a patient's FVC falls below 80% with symptoms or below 50% without symptoms, the situation warrants the implementation of non-invasive ventilation.
Panel Recommendation 5: Sleep-related Breathing Disorders
Panel recommendation number five pertains to the intersection of neuromuscular diseases and sleep-related breathing disorders. The panel highlights the utilization of non-invasive ventilation as a treatment approach for individuals with neuromuscular diseases facing sleep-related breathing disorders.
Panel Suggestions:
Based on limited available data, transcutaneous and end-tidal CO2 measurements exhibited potential in identifying hypoventilation and initiating as well as managing NIV. Home-based overnight capnography emerged as a practical option and criterion for initiating respiratory support. Furthermore, the panel advises referencing the criteria outlined by the American Academy of Sleep Medicine (AASM). Familiarity with these criteria is valuable, and considering a sleep course might be beneficial if you are not already acquainted. The AASM criteria pertain to sleep disorder-related hypoventilation in adults. It is also important to note that the European Respiratory Society (ERS) offers its own guidelines and recommendations tailored specifically for pediatric patients. While the panel endorses the use of non-invasive ventilation, they emphasize the significance of aligning with the appropriate guidelines for comprehensive and effective treatment.
Panel Recommendation 6: Respiratory Parameters for NIV
When it comes to determining the parameters for non-invasive ventilation in patients with neuromuscular diseases, the panel suggests employing diagnostic tests such as FVC, MIP/MEP, ONO, or evidence of sleep-disordered breathing or hypoventilation on polysomnography to predict the timing of NIV initiation. Additionally, the panel proposes using parameters observed on the polygraph to predict the appropriate timing for initiating non-invasive ventilation in sleep-related breathing disorders or hyperventilation cases. Polysomnography is not deemed obligatory for adult NMD patients under NIV management if PFT or ONO criteria validate its use. In situations where concerns linger regarding whether PFT and clinical assessments adequately address potential complications like hypoventilation, sleep testing can prove beneficial.
Panel Suggestions:
The panel emphasizes that initiating non-invasive ventilation does not necessarily require polysomnography for adults. Instead, relying on the criteria established through pulmonary function tests (PFT) alone can be sufficient for guiding the decision. Notably, ONO presents distinct advantages of being cost-effective and easy to repeat. ONO serves dual roles: evaluating nocturnal desaturation to levels below 88% for a 5-minute interval, potentially qualifying NMD patients for NIV devoid of OSA indications, and monitoring NIV effectiveness by ensuring oxygen saturations exceed 90% during over 90% of the NIV recording. This shift in focus minimizes the necessity for polysomnography in the initiation process.
Panel Recommendation 7: Individualizing NIV Treatment
Recommendation number seven addresses the treatment approach for patients with neuromuscular disease necessitating non-invasive ventilation. The panel highlights the importance of tailoring the non-invasive ventilation treatment to align with individual ventilation goals, as It is not a one-size-fits-all solution.
Panel Suggestions:
The panel recommends adjusting variables like mode selection, inspiratory time, and inspiratory/expiratory pressures. While no specific mode is favored over others, It is crucial to select a mode and then fine-tune parameters like inspiratory time and pressures to meet the patient's unique ventilation objectives. This adaptation process varies on an individual basis, taking into account the broader hospital context, yet each patient's distinct needs must be prioritized when making these determinations.
The panel also suggests that implementing a backup respiratory rate could enhance patient-ventilator synchrony and improve gas exchange. It is important to note that patients with bulbar impairment might face challenges in tolerating non-invasive ventilation. Furthermore, the panel advocates for ongoing assessment encompassing factors such as sleep quality, digital data analysis, leak management, oximetry, and capnography. This comprehensive approach ensures an effective treatment strategy and encompasses the multifaceted aspects of patient care.
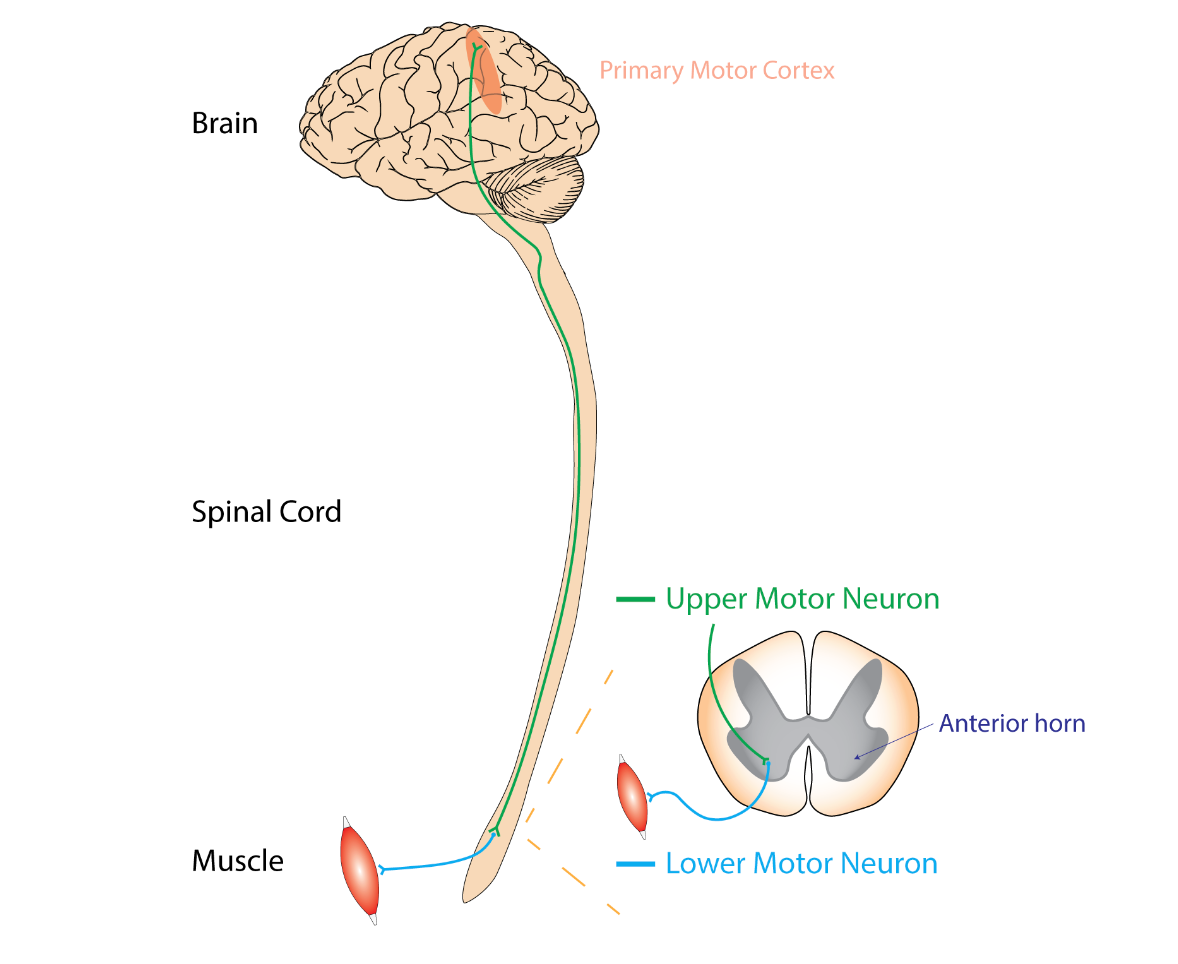
Figure 4. Diagram of bulbar neuron impairment (Rcchang16, CC BY-SA 4.0 via Wikimedia Commons).
The topic of bulbar impairment is a crucial consideration in non-invasive ventilation. Bulbar impairment in Figure 4, often referred to as bulbar palsy, results from conditions affecting the lower cranial nerves, typically the seventh and eighth nerves. This condition can lead to speech deficits due to paralysis affecting these nerves.
In the accompanying visual in Figure 4, you can observe the damage to the lower motor neuron, which stems from the primary motor cortex of the brain. This damage can significantly impact speech functions. It is important to understand that non-invasive ventilation might not be a suitable solution when we refer to bulbar impairment due to the nature of the impairment itself. Certain populations, particularly individuals with bulbar dysfunction, may face challenges in performing PFTs. Additionally, daytime PFTs can serve as a valuable indicator of sleep-related hypoventilation, exhibiting a correlation with durations of oxygen saturation ≤ 90% and arterial blood gas Pco2 levels ≥ 45 mm Hg.
Diagnosing bulbar impairment is the realm of medical professionals, but It is essential for us to comprehend that non-invasive ventilation would not effectively address this condition. This understanding is pivotal for making informed decisions about the appropriate course of action in such cases.
Panel Recommendation 8: Bulbar Function
Recommendation number eight addresses the use of non-invasive ventilation in patients with preserved bulbar function who are affected by neuromuscular diseases. In such cases, the panel advises considering the utilization of mouthpiece ventilation (MPV) during daytime hours, serving as an adjunct to the traditional nocturnal mask ventilation.
Panel Suggestions:
While mouthpiece ventilation has been employed to delay the need for mechanical ventilation in neuromuscular diseases, it is important to acknowledge that its effectiveness could be limited by bulbar symptoms in some cases. If non-invasive ventilation is necessary, opting for mouthpiece ventilation is recommended. Various designs exist for mouthpiece ventilation, which is distinct from conventional ventilation due to the absence of intubation. Patients are fitted with a mouthpiece instead. This method is endorsed as a viable approach in cases where preserved bulbar function exists.
Panel Recommendation 9: Mechanical Ventilation
In cases where patients with neuromuscular diseases experience non-responsive non-invasive ventilation and their bulbar function deteriorates, leading to issues like aspiration, inadequate cough, and declining lung function, adopting home mechanical ventilation via tracheostomy becomes a viable alternative. The panel strongly recommends initiating mechanical ventilation as early as feasible, alongside careful consideration of care objectives. This move is particularly pertinent if non-invasive ventilation proves ineffective.
Panel Suggestions:
The positive impacts of MPV remain uncertain, yet they might encompass postponing or circumventing tracheostomy, enhancing speech, augmenting cough efficacy, and refining the synchronization of breathing and swallowing. Given the limited evidence, the equilibrium between risks and advantages likely tips in favor of the intervention, although the certainty of evidence remains very low. Even when confronted with worsening bulbar function, aspiration, ineffective cough, and declining lung function, the panel suggests transitioning to regular mechanical ventilation. The key is to ensure appropriate ventilation and care despite these challenges.
Furthermore, the panel advocates for optimizing secretion management and airway clearance, tailoring these approaches according to patient preferences, treatment objectives, quality of life factors, and available resources. This comprehensive strategy assists in making informed decisions regarding patient care. This emphasis on personalized care accounts for various facets and aligns with the overall care approach.
Panel Recommendation 10: Sialorrhea Management
Moving on to panel recommendation number ten, which addresses sialorrhea in patients with neuromuscular diseases. In such cases, the panel suggests commencing with a therapeutic trial of an anticholinergic medication as the initial treatment option. This approach serves as the primary line of therapy for managing sialorrhea in these patients. Sialorrhea is a prevalent issue in NMD, especially notable in ALS, and can elicit significant distress, leading to a diminished quality of life. It also amplifies the risk of aspiration and pneumonia due to challenges in swallowing, safeguarding the airway, and fostering effective coughing. Consequently, the panel strongly recommends initiating treatment with a trial of anticholinergic agents, which offer affordability and accessibility.
Panel Suggestion:
The panel recommends starting with a trial of oral anticholinergic medication. We are familiar with anticholinergic medicines like DuoNeb and Atrovent, which block cholinergic responses. This action not only addresses their intended effects but also has a secondary effect of reducing secretions. This dual function is important to grasp when considering their use for conditions like sialorrhea.
Sialorrhea
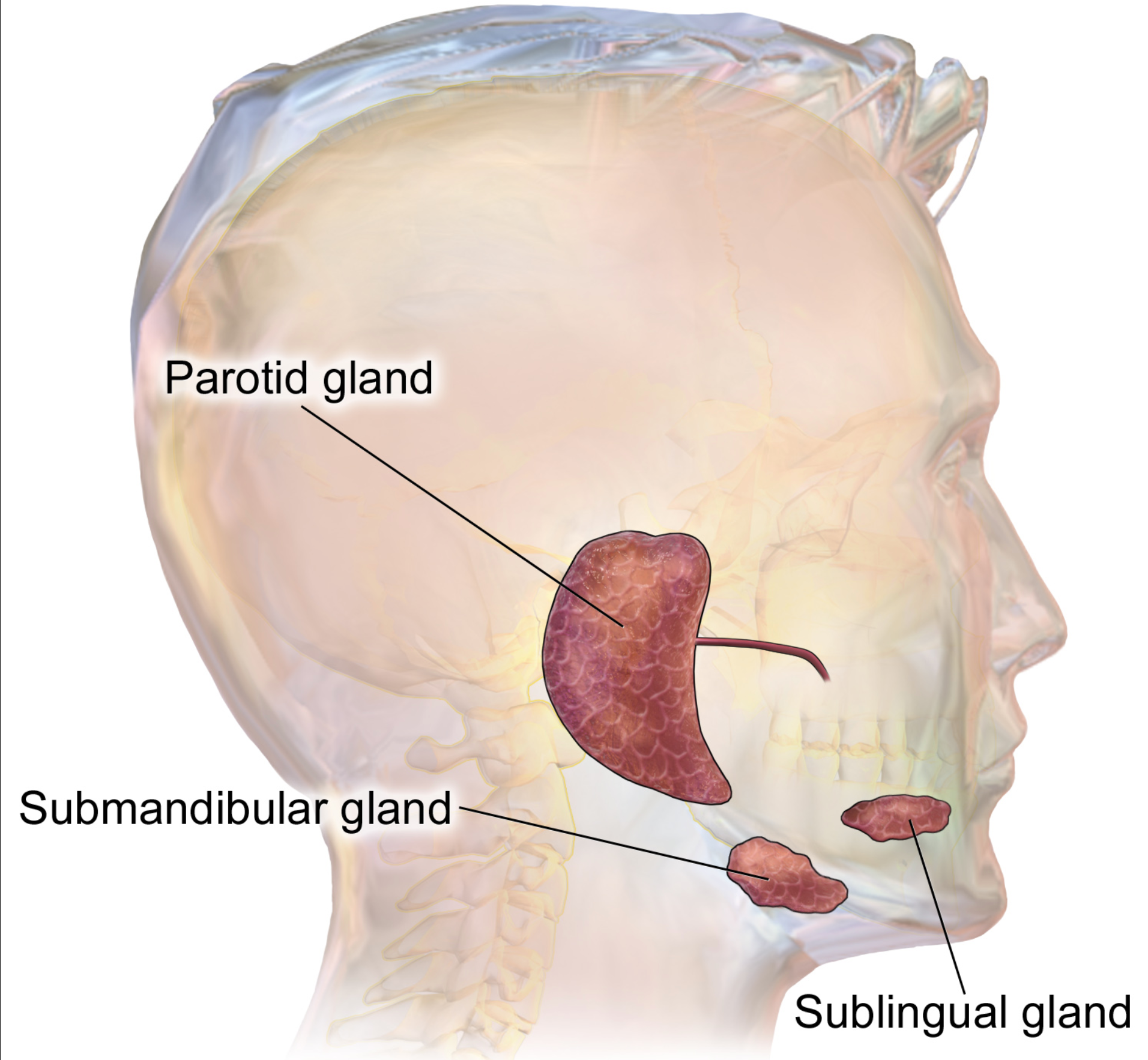
Figure 5. Diagram of salivary glands. (Blausen Medical WikiJournal of Medicine, CC BY 3.0).
Sialorrhea, also known as drooling, is characterized by the presence of excessive saliva beyond the lip margin seen in Figure 5. This condition can manifest due to various neurologic disorders, including ALS, cerebral palsy, Parkinson's disease, and medication side effects. In children, cerebral palsy is the most frequent cause of sialorrhea. Anticholinergic medications can be beneficial for managing excessive drooling in children as they offer a drying effect. Beyond its role in this context, anticholinergics are known to block acetylcholine, which can affect bodily functions, including heart rate regulation.
In the accompanying image (Figure 5), you can observe the various affected areas when someone experiences sialorrhea. The buildup of excessive secretions can pose significant challenges. While anticholinergic drugs are a valuable option, they aren't the sole approach available for addressing this issue.
Panel Recommendation 11: Botulinum toxin (BT) Therapy for Salivary Glands
For patients with NMB and sialorrhea who have an inadequate response or are intolerant of the side effects of anticholinergic therapy, the panel suggests using Botulinum toxin (BT) or Botox.
Panel Suggestion:
A potential alternative for individuals facing challenges with anticholinergic therapy due to side effects is Botulinum Toxin (BT) treatment targeted at the salivary glands. Some individuals find it difficult to tolerate anticholinergic agents because they can impact heart rate regulation, accelerating the heart rate by blocking the slower processes. In cases where anticholinergic therapy is not suitable, the panel also suggests considering the use of Botox injections for the salivary glands. This option stands as a valid panel recommendation for addressing sialorrhea.
Panel Recommendation 12: Salivary Gland Radiation
Panel recommendation number 12 addresses the same concern with sialorrhea. It pertains to patients with neuromuscular disease and sialorrhea who face an inadequate response to or cannot tolerate the side effects of anticholinergic therapy. Alongside the option of using Botox injections, the panel recommends considering salivary gland radiation as an alternative. This treatment approach is more comprehensive and specific to the disease context.
Neuromuscular diseases can present significant challenges, particularly when accompanied by uncontrollable drooling due to sialorrhea. In such cases, exploring advanced options like salivary gland radiation underscores the gravity of the issue and the need for tailored interventions to enhance the patient's quality of life.
Panel Suggestion:
The panel suggests using salivary gland radiation as an option when anticholinergic therapy or Botox injections do not yield the desired results. This approach serves as an additional avenue for managing sialorrhea in patients with neuromuscular disease, particularly when other interventions prove ineffective. The aim is to provide a comprehensive range of options to address this challenging condition and improve patients' well-being.
Panel Recommendation 13: Glossopharyngeal Breathing for Lung Volume Recruitment (LVR) and Airway Clearance
For patients with NMB and hypoventilation, the panel recommends Glossopharyngeal Breathing (GPB) for lung volume recruitment (LVR). This technique is preferred due to its low cost and the patient's ability to perform it independently. The technique requires minimal training or assistance.
Panel Suggestion:
For patients experiencing hypoventilation, an approach to consider is glossopharyngeal breathing (GPB) for lung volume recruitment. This technique aims to counter the development of atelectasis by recruiting alveoli. In essence, GPB is a positive pressure breathing technique suggested by the panel to aid failing respiratory muscles, keeping in mind the context of muscle weakness.
The glottis plays a key role in this method, trapping air in the lungs while a gulp of air is drawn in. While numerous breathing techniques exist, GPB emerges as a valuable option highlighted by the panel for its potential to enhance lung function and counter the challenges associated with muscle weakness and hypoventilation.
Glossopharyngeal Breathing (GPB)
Glossopharyngeal breathing offers a distinct approach to enhance voice and cough effectiveness. This technique focuses on trapping air within the lungs through a stepwise process. Beginning in a relaxed position, the patient sequentially employs the glottis to retain air while taking a gulp of air, followed by a passive exhalation. This method effectively maintains lung expansion and function by harnessing the glottis to hold air, ensuring optimal lung capacity and assisting in voice and cough improvement. This recommendation presents an alternative breathing technique to optimize lung function and address issues like muscle weakness.
Panel Recommendation 14: Reduced Cough
Panel recommendation number 14 addresses patients with neuromuscular diseases who experience diminished cough. Recognizing the critical significance of effective coughing, the panel suggests the adoption of manually assisted cough techniques independently or added to other modalities such as LVR. These techniques encompass various approaches to aid patients in achieving a productive cough.
Panel Suggestion:
The panel recommends employing these cough techniques independently or integrating them with other modalities, like lung volume recruitment (LVR). Essentially, any effective cough technique that can be utilized is encouraged. The panel emphasizes the value of attempting various assisted cough techniques, including those previously employed, to enhance the patient's ability to clear their airways effectively.
Panel Recommendation 15: Reduced LVR
Panel recommendation number 15 pertains to patients with neuromuscular disease experiencing diminished lung volume recruitment (LVR) and cough capabilities. In such cases, the panel advises the utilization of standard LVR techniques employing a handheld resuscitation bag or a mouthpiece.
Panel Suggestion:
The technique itself is cost-effective, yet it does require training and assistance. Notably, manual-assisted coughing proves more effective when combined with volume recruitment or expiratory cough assist. The essence of this technique lies in utilizing a handheld resuscitation bag or a mouthpiece to facilitate coughing. However, it's essential to provide patient training for proficient execution. This recommendation emphasizes a comprehensive approach to enhance lung function and cough effectiveness.
Panel Recommendation 16: Reduced Cough Effectiveness
For patients with neuromuscular disease experiencing compromised cough effectiveness that is not responsive to alternative techniques, the panel recommends integrating a regular mechanical insufflation-exsufflation (MI-E) cough assist device. You might be familiar with these devices, which aid in clearing secretions.
Panel Suggestion:
The panel's suggestion to employ MI-E devices underscores their importance in addressing excessive secretions and the associated risks posed by muscle weakness. The accumulation of excess secretions can lead to serious issues, including atelectasis. Consequently, the recommendation urges the use of cough assist devices like MI-E to mitigate these concerns. While various types of devices are available, the one shown here represents the approach. Assisting individuals with coughing is vital for maintaining respiratory health, whether they have neuromuscular diseases or not. However, providing assistance becomes even more critical for those with neuromuscular conditions due to the challenges they face. Handheld devices play a significant role in aiding individuals in effectively clearing their airways and managing secretions. The goal is to ensure optimal lung function and minimize the potential complications associated with muscle weakness and compromised cough effectiveness.
Panel Recommendation 17: Secretions
Panel recommendation number 17 focuses on secretion management, introducing various devices. For patients experiencing challenges in clearing secretions due to neuromuscular diseases, the panel suggests the utilization of High-Frequency Chest Wall Oscillation (HFCWO), commonly known as vest therapy, for secretion mobilization. In addition, HFCWO is suggested to be combined with airway clearance therapies such as cough assistance or LVR.
Panel Suggestion:
This approach is recommended and can be combined with other airway clearance devices like the cough assist or lung volume recruitment (LVR). These familiar devices, commonly used in our practice, gain enhanced significance in the context of neuromuscular diseases. Notably, the panel recommends using the vest as part of a comprehensive strategy to ensure effective coughing and secretion management. The primary objective is to prevent potential complications that may arise when patients struggle to clear their secretions effectively.
The panel has devised a flowchart (click on the link to access or refer to the course handout) for initiating non-invasive ventilation (NIV) in patients with NMD who exhibit respiratory failure symptoms. Beginning at the top left with the neuromuscular disease, the flowchart outlines a structured process. This includes recommendations for performing PFTs every six months. If criteria are met, progression to the next phase occurs. In instances where criteria are not met, scheduling a follow-up PFT in six months is suggested. Monitoring for current symptoms is essential. If the criteria are met, NIV initiation can be considered. Additionally, ABG and sleep study results are considered, demonstrated in the flowchart.
This flowchart effectively breaks down the discussed criteria, including forced vital capacity thresholds and symptoms, providing a visual representation that aids in understanding and retention. Consider utilizing this informative slide as a resource to reinforce the concepts discussed in our conversation. While not mandatory, this new research offers valuable insights into managing patients with neuromuscular diseases.
Clinical Scenarios
Question 1:
When caring for a patient with an NMD, the panel suggested that a PFT be done at least how often?
A. Once a year
B. Every 3 months
C. Every 6 months
D. PFTs are not recommended for NMD patients
Rationale:
For patients with neuromuscular diseases prone to respiratory failure, the recommendation is to schedule evaluations at least every six months, aligned with the progression of the particular neuromuscular condition. When discussing Pulmonary Function Tests (PFT), it is crucial to focus on Forced Vital Capacity, Slow Vital Capacity, as well as assessing respiratory muscle strength through Maximum Inspiratory Pressure (MIP) and Maximum Expiratory Pressure (MEP). Clear comprehension of these elements is essential as they form an integral aspect of the treatment protocol for patients afflicted by neuromuscular disorders.
Question 2:
For patients with systemic NMD who have a normal PFT and overnight oximetry (ONO), what is suggested by the panel to assess whether noninvasive ventilation (NIV) is needed?
A. Polysomnography
B. Chest X-Ray
C. ECG
D. EEG
Rationale:
In the case of patients affected by systemic neuromuscular diseases who exhibit normal overnight oximetry results but require assessment for the necessity of non-invasive ventilation, the recommended approach by the panel involves polysomnography. Polysomnography serves as a diagnostic tool to determine the suitability of non-invasive ventilation for systemic patients with normal Pulmonary Function Test (PFT) results. Therefore, when patients present with normal overnight oximetry data, the appropriate course of action involves conducting a polysomnography, commonly referred to as a sleep study, to ascertain the potential requirement for non-invasive ventilation.
Question 3:
For the treatment of patients with NMD and sleep-related breathing disorders, the panel suggests which of the following?
- Tracheostomy
- Bronchodilator therapy
- PFT
- Noninvasive Ventilation (NIV)
Rationale:
When addressing the treatment approach for patients with neuromuscular diseases who exhibit sleep-related disorders, and considering options such as tracheostomy, bronchodilator therapy, Pulmonary Function Tests (PFT), or non-invasive ventilation, the recommended course of action is to opt for non-invasive ventilation.
This decision aligns with the criteria established by the American Academy of Sleep Medicine (AASM) for adults and the European Respiratory Society's guidelines for pediatric patients experiencing sleep-related breathing hypoventilation. As per these guidelines, once patients meet the specified criteria during evaluations, it becomes appropriate to initiate non-invasive ventilation. It is noteworthy that the decision to employ non-invasive ventilation applies to both adult and pediatric patients, depending on which set of guidelines is applicable to the specific case.
Question 4:
For patients with NMD and sialorrhea, the panel suggests the use of which treatment?
- Sympathomimetic bronchodilator medication
- Anticholinergic medications
- Steroidal medications
- Nonsteroidal anti-inflammatory drug
Rationale:
In the scenario where a patient with a neuromuscular disorder is experiencing issues with excessive drooling and impaired fluid control, the recommended solution is the utilization of anticholinergic medication. The panel suggests an initial trial of a cost-effective oral anticholinergic medication as a suitable intervention.
When considering anticholinergic drugs, examples such as Atrovent and Spiriva are relevant. These medications work to address the symptoms associated with excessive drooling and inadequate fluid control caused by neuromuscular disease.
Question 5:
After evaluating patients with NMD and difficulties with secretion clearance, the panel recommends using which for secretion mobilization?
- Suctioning with a large catheter
- High-Frequency Chest Wall Oscillation (HFCWO)
- Incentive spirometer
- Tracheostomy
Rationale:
To facilitate the movement and clearance of secretions, a strategy often employed is secretion mobilization. The term "mobilization" signifies movement in this context. An effective solution, as recommended by the panel, is the utilization of high-frequency chest wall oscillation, commonly known as the vest. This approach has been suggested by the panel for enhancing secretion mobilization.
Furthermore, it is worth noting that incorporating vests, like the high-frequency chest wall oscillation device, can be enhanced by combining them with other airway clearance devices. This combined approach aligns with the panel's suggestions for optimizing the effectiveness of secretion mobilization techniques.
Summary
Our discussion has revolved around neuromuscular diseases and their respiratory management. The panel's recommendations have been formed based on an amalgamation of 128 studies. These recommendations are categorized as graded and consensus-based statements.
These guidelines underscore the challenges posed by limited research, largely reliant on observational data from ALS patients. Our primary focus has been on pulmonary function assessment. Low-cost interventions have been highlighted, particularly forced vital capacity (FVC), slow vital capacity, maximum inspiratory and expiratory pressures, including SNIP (nasal inspiratory pressure), and peak cough flow. These parameters should be considered in patients with neuromuscular diseases, bearing in mind clinical practice variances.
Furthermore, the panel endorsed the utilization of pulmonary function tests (PFTs) at least every six months, based on the specific neuromuscular disease progression. The essentials include FVC, MIP, and SNIP. For those with normal PFTs but sleep-related disorders, polysomnography is recommended to assess the need for non-invasive ventilation.
In the context of chronic respiratory failure in neuromuscular disease patients, non-invasive ventilation is advocated. The criteria include FVC dropping below 80% of predicted with symptoms or below 50% without symptoms, as well as SNIP/MIP falling below 40 cmH2O or the presence of hypercapnia.
Polysomnography aids in predicting the timing of non-invasive ventilation. In specific cases, PFT criteria alone suffice. Non-invasive treatment aligns with hospital goals, emphasizing an individualized approach. For patients with bulbar impairment, toleration of non-invasive ventilation can be challenging. Mouthpiece ventilation is considered, along with nocturnal mechanical ventilation for those with preserved bulbar function. Management includes anticholinergic medication or Botox for sialorrhea. In severe cases, salivary gland radiation might be necessary.
For lung recruitment, glossopharyngeal breathing can be explored. Reduced cough capacity necessitates assisted cough techniques, potentially utilizing insufflation-exsufflation devices. Secretion mobilization is vital, with options including vests and PEP devices to aid in preventing complications from impaired cough reflexes. This comprehensive approach, from PFTs to non-invasive ventilation, underscores the commitment to aiding patients facing neuromuscular diseases.
References
Berry, R. B., Chediak, A., Brown, L. K., Finder, J., Gozal, D., Iber, C., Kushida, C. A., Morgenthaler, T., Rowley, J. A., Davidson-Ward, S. L., & NPPV Titration Task Force of the American Academy of Sleep Medicine (2010). Best clinical practices for the sleep center adjustment of noninvasive positive pressure ventilation (NPPV) in stable chronic alveolar hypoventilation syndromes. Journal of clinical sleep medicine: JCSM : official publication of the American Academy of Sleep Medicine, 6(5), 491–509.
Khan, A., Frazer-Green, L., Amin, R., Wolfe, L., Faulkner, G., Casey, K., Sharma, G., Selim, B., Zielinski, D., Aboussouan, L. S., McKim, D., & Gay, P. (2023). Respiratory Management of Patients With Neuromuscular Weakness: An American College of Chest Physicians Clinical Practice Guideline and Expert Panel Report. Chest, S0012- 3692(23)00353-7. Advanced online publication.
Silva, I. S., Pedrosa, R., Azevedo, I. G., Forbes, A. M., Fregonezi, G. A., Dourado Junior, M. E., Lima, S. R., & Ferreira, G. M. (2019). Respiratory muscle training in children and adults with neuromuscular disease. The Cochrane database of systematic reviews, 9(9), CD011711.
Warren, V.C. (2002). Glossopharyngeal and neck accessory muscle breathing in a young adult with C2 complete tetraplegia resulting in ventilator dependency. Physical
therapy, 82 6, 590-600.
Citation
Reed, D. (2023). Respiratory management of patients with neuromuscular weakness series: review recommendations best practice. Continued.com - Respiratory Therapy, Article 192. Available at www.continued.com/respiratory-therapy
